Wayne Swirnow, President
Level III Certified Infrared Thermographer
Infrared Imaging Services, LLC
PO Box 39Burlington, NJ 08016Ph: 845-641-5482 www.infraredimagingservices.com
Abstract
It has long been known that all real world objects above zero Kelvin radiate infrared energy. Emissivity is the term used to describe the rate at which the surface of an object can transfer radiated energy. Experienced thermographers are well aware of emissivity and its relationship to reflectivity, but what exactly is it which makes the surface property of one material a good emitter and another a poor emitter? This paper will explore the topic of emissivity and will examine the surface properties of materials to explain the difference.
Discussion
Emissivity – Definition
Emissivity is the term used to define a surface’s efficiency for radiating electromagnetic wave energy in a defined waveband at a given temperature.
It is the rate at which the surface of an object can transfer energy via radiation.
Any surface above absolute zero will radiate some energy, more than 0% and less than 100%.
Emissivity
Emissivity is casually summarized as “shiny things reflect infrared energy and do not radiate well, non-shiny surfaces emit well and do not reflect as much”.
Low emissive surfaces are more prone to measurement and observational errors because they do not radiate well.
The question is “What causes shiny surfaces not to radiate well”?
Kirchhoff and his Laws
Gustav Robert Kirchhoff (12 March 1824 – 17 October 1887)
A German physicist who contributed to the fundamental understanding of electrical circuits, spectroscopy, and the emission of blackbody radiation by heated objects.
For an arbitrary body emitting and absorbing thermal radiation in thermodynamic equilibrium, the emissivity is equal to the absorptivity.
Before Kirchhoff’s law was recognized, it had been experimentally established that a good absorber is a good emitter, and a poor absorber is a poor emitter. Therefore, a good reflector must be a poor absorber.
But how does this work?
Why don’t shiny surfaces absorb well?
Why, if a shiny metal is hot, can it not radiate infrared waves EM well, just because it is shiny?
What are the element(s) of a surface’s property that enable it to emit or not emit infrared radiation, and how is this related to it being reflective?
Electromagnetic Wavelengths
A wavelength has a physical size or period and is defined as the distance between two like points completing one full cycle of the waveform.
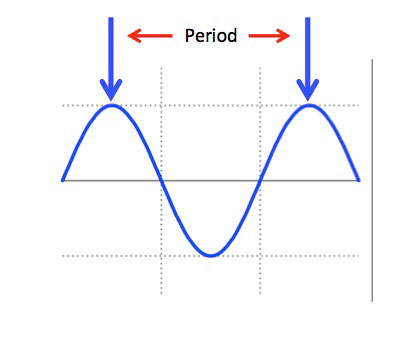
Relative Wavelength Size
Infrared is EM energy composed of particles called photons, traveling in waves. All EM waves have a Frequency a therefore a Wavelength or Period
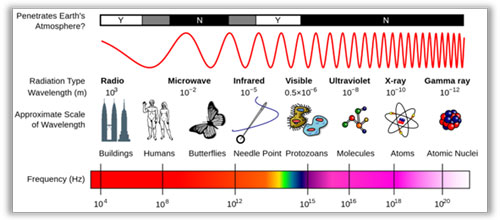
IR Wavelength Size
Short wave infrared wavelengths are 4X shorter than longwave infrared wavelengths.
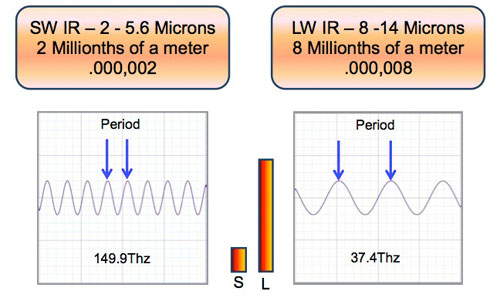
A human hair is about 50 microns
Properties of Metals
Atoms in metals easily lose outer shell electrons and become positively charged ions called cations.
The “sea” of free flowing electrons is free to move within the solid structure allowing the metal to easily conduct heat and electricity.
The solid characteristic is produced by electrostatic interactions between each atom and the sea of electrons. This is called the Metallic Bond.
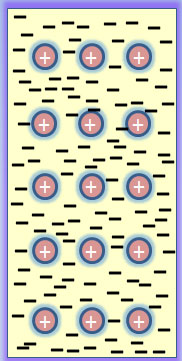
When photons traveling in waves hit the surface of a metal, they induce oscillations in the surface electrons (skin effect) causing each electron to wiggle and emit a photoelectron.
Some energy of the incident photon is required to free the electron from the surface, and the remaining energy becomes the kinetic energy of the freed photoelectron. We call this released photo electron wave a “reflection”.
Because the free valance electrons are not strongly attached to the atomic lattice, when hit by the incident photons, they do not transfer this energy to the lattice. The skin effect is present, the incident wave is “reflected”, and the metal does not absorb the incident energy.
This is why polished metals at low temperatures cannot efficiently absorb incident infrared energy.
Due to the weak connections between the cations and free electrons, when mechanically warmed (i.e., resistive electrical connection), the cations cannot transfer enough energy to cause surface electrons to oscillate and emit photo electrons.
Emissivity varies with temperature and wavelength. An increase in the temperature is accompanied by a decrease in the wavelength according to the Planck distribution.
When warmed to higher temperatures the oscillation frequency increases, wavelength gets shorter and there is sufficient energy imparted to the surface electrons to emit energy in the visible spectrum, i.e. “Red hot”.
Specular Reflection – Metals
For a smooth surface, the photons traveling in waves stay parallel to each other and there is an identical reflected image, “specular” (mirror like) reflection.
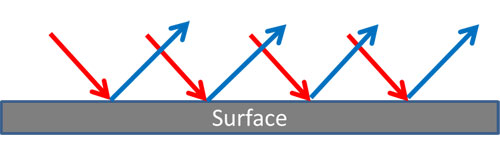
Diffuse Reflection – Metals
If the surface of a metal is rough (but still shiny) there is a diffuse reflection. The incident wave photons are reflected at many angles.
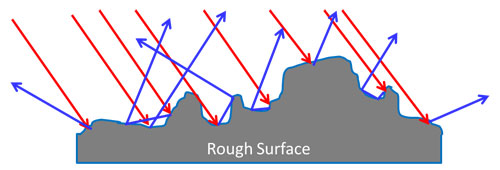
Reflection – Non Metals
In non-metal materials lacking the free electrons, the photons can penetrate into the material.
The photon waves induce small oscillations of the individual atoms causing each particle to radiate a small secondary wave. If the surface interface is smooth, the reflection is specular. If not smooth, it is a diffuse reflection.
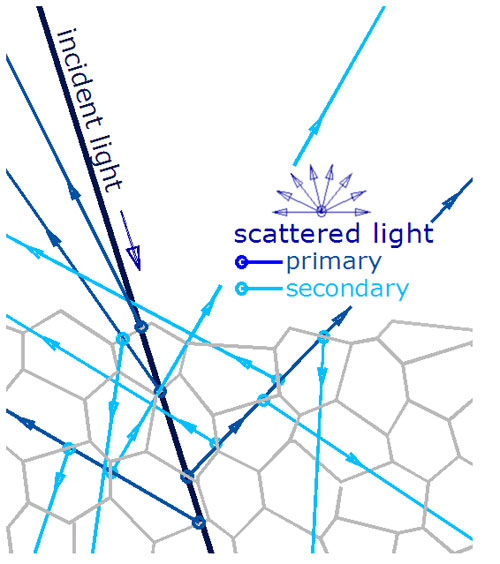
Diffuse and Specular Reflection – Non Metals
Specular reflection needs a flat surface, but a flat surface does not prevent diffuse reflections, an example would be polished granite.
Photons penetrate the surface and have multiple reflections from multiple particle interfaces, creating a series of primary and scattered rays in random directions.
There will be some specular reflections and diffuse reflections.
Diffuse Reflections – Lambert’s Law
Johann Heinrich Lambert, French and German mathematician, 1728-1777.
In diffuse reflections, the EM energy is reflected in many angles according to Lambert’s emission law.
Emitted power from a given area element is reduced by the cosine of the emission angle. This gives us the effect of lower emissivity values as the angle increases.
Emissions of photons are greatest perpendicular to the normal plane and are proportional to the cosine of the angle between the observer and the flat surface.
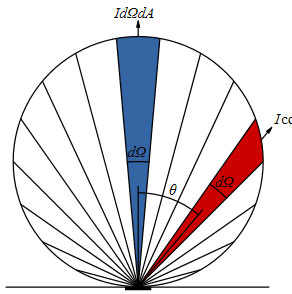
Lambert’s cosine explains why imaging from a perpendicular angle gives the most accurate results.
Emissive Surface Properties – General
Nonmetallic (high E) materials with surface electrons strongly coupled to atoms below them can absorb IR energy.
The incident wave hits the surface electrons causing them to wiggle, thus increasing their energy. Being well coupled to the internal atomic structure, the energy is absorbed (conducted) into the material.
The material re-radiates the same frequency and amount of energy (minus reflections) as the incident absorbed energy.
As Kirchhoff said, “For an arbitrary body emitting and absorbing thermal radiation in thermodynamic equilibrium, the emissivity is equal to the absorptivity.”
This is why emissive materials both absorb and emit energy well.
Wavelengths vs Emissivity/Absorptivity
Antennas have a physical length equal to a fractional multiple of the signal wavelength or period.
When the antenna length matches the fractional period of the wavelength (i.e. 1/2 wave), it resonates at the signal frequency and can transmit/receive EM radiation.
Antenna length is proportional to the EM signal wavelength:
-
Lower frequencies = Longer antennas
-
Higher frequencies = Shorter antennas
-
Very high frequencies = Very small antennas
Energy in Waves
Wavelengths of SW and LW infrared – let’s review by showing the relative size of the wavelengths.
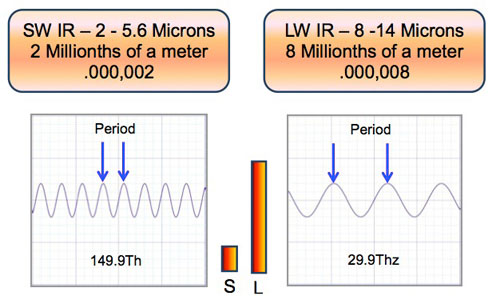
Wavelengths and Surface Texture – Non Metals
Surfaces are not perfectly smooth; all have some texture, nooks and crannies. Surface texture size is analogous to antenna length. If the EM wavelength is smaller than the texture size, it fits into the texture, bounces around within it and can be absorbed.
-
Absorptivity is higher
-
Emissivity is higher.
If the EM wavelength is larger than the texture size, it does not fit into the texture well and energy is reflected at that EM wavelength.
-
Absorptivity is lower
-
Emissivity is lower
This contributes to why emissivity changes with different material surfaces.
Wavelength and Emissivity
Smooth surfaces have smaller texture and tend to have more specular reflection at longer wavelengths because the wavelengths do not fit into the texture structure.
Rough surfaces tend to have a more diffuse reflection and can interact with longer wavelengths as well as shorter wavelengths.
Smooth surfaces radiate / absorb better at shorter wavelengths.
Surface texture size is one factor affecting the wavelength a material will reflect / absorb at therefore affecting its emissivity at that wavelength.
This is why smooth surfaces are best viewed with shortwave IR imagers
Surface Texture and Emissivity
Emissivity, reflectivity, and absorptivity are all related and primarily a property of a material’s surface.
Changing the surface properties of a material will change the emissivity, absorbance and reflectivity of that surface. The surface modifier may increase emissivity and absorbance by reducing reflectivity conducting more IR energy into a low emissivity material, or it may decrease emissivity and absorbance, by increasing reflectivity and preventing a material from radiating infrared energy.
This is why surface modifiers such as electrical tape, paint, and shiny metal cladding, increase or decrease a material’s emissivity.
*******************
Infrared Imaging Services LLC acknowledges the internet authors, scientists, Wikipedia references and all others who contributed and posted materials on the topic of reflectivity and emissivity.
A special thank you to Physics Professor Saeed Safaie, for his consultation on this presentation.



